Written by Daniel Mannina
April 15, 2020
KITCHEN SCIENCE
Matter is everything that has mass and takes up space – including water. From freezers to faucets to stoves – water exists in three states in the kitchen.
BASICS
This exercise requires water, three 16-ounce glasses, a small pot, a stove and a freezer. Follow the instructions below to experience water in each of its forms.
- Measure 16 ounces of water and pour it into three clear glasses.
- Place one glass to the side and label it “liquid.”
- Place the second glass of water in the freezer* and allow it time to freeze. Then remove it from the freezer, label it “solid” and place it next to the liquid form.
- Now, pour the water from the third glass into a small pot on the stove; allow it time to boil. Once it starts boiling, label an index card with “gas” placing it adjacent to the stove in a safe place; discuss what was observed.**
WHAT HAPPENED?
At the beginning of the experiment, all three glasses had the same shape and volume. But as each was manipulated, its physical characteristics started to change.
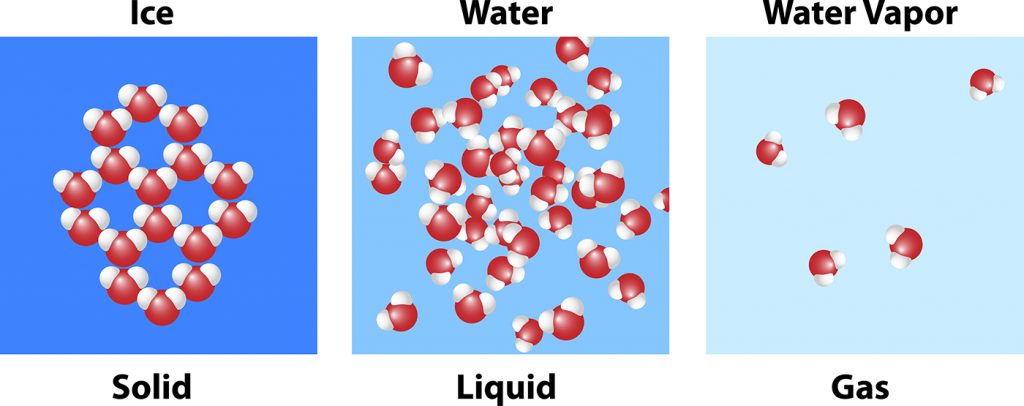
- Liquid state – The water in its liquid form maintained its volume, while its shape was indefinite, allowing the pouring from one glass to another. The molecular bonds were looser, which allowed the movement.
- Solid state – When the water was frozen at 32 degrees Fahrenheit, it solidified and maintained a definite shape and volume with a very tight molecular bond.
- Gaseous state – The water boiling on the stovetop at 212 degrees Fahrenheit became a gas leading it to have no definite shape or volume. The molecular bonds became chaotic as the steam rose.
WHAT IS HAPPENING?
One great way to evolve this for older ages is to create kitchen “H20 molecules.” This exercise requires toothpicks and assorted round fruits/vegetables of varying sizes.
- Create water molecule model: Grab two smaller items like grapes (simulating the hydrogen atoms) and attach them with toothpicks to something like a potato, apple or orange (simulating the oxygen atom). This created a water molecule. Repeat this step until there are three molecule models.
- Simulate a solid: Place all three molecule models closely together, tightly so they cannot move. This shows the type of close bonds that solids have.
- Simulate a liquid: Now, spread them farther apart, loosely touching. This shows the type of loose bonds that liquids have.
- Simulate a gas: Finally, take all three modes and simply move them around each other quickly without touching. This shows the type of chaotic bonds that gases have.
To break it down: the shape of the actual molecules and spacing between elements remains the same, while way they interact with the other water molecules changes. The changes occur through the application of physical forces, similar to the first activity.
In this experiment, the smaller items represented the positively charged hydrogen atoms and the bigger item the negatively charged oxygen atom. The positive side of one attracted to the negative side of the other.
In the solid state (ice) these molecules were packed closely together and the +/- connections formed a lattice work pattern – essentially interconnected hexagons. This set crystal pattern leaves room for open space even when the molecules are packed tightly together. That is the reason why water, unlike most other types of matter, is actually less dense in its solid state than the liquid state. Fun fact: that’s why ice floats!
*Make sure the glasses placed in the freezer can withstand frigid temperatures.
**Make sure children are a safe distance from potential boiling water splashes and rising hot steam.
Share your successes, failures and adjustments along with the final product with @FIUCASE on social media.
Central to every house there, is no better STEM classroom than the kitchen. From chemistry to biology to physics and beyond investigate the art and science behind culinary creations and everyday life. Follow FIU@Home on CASE News for more kitchen science.